Table of Contents (click to expand)
To calculate empirical formula of a compound, find the mass of each element present in the compound and convert it to moles, calculate the individual mole ratios and then write out the empirical formula.
There are numerous ways in which information regarding the molecular structure and composition of a chemical compound can be exhibited. These include the commonly used molecular formula, which shows the number of atoms of each element in the compound, as well as the structural formula, which illustrates the arrangement and bonds of the different atoms in a compound.
Another very important form of communicating information regarding a compound is with its empirical formula. The empirical formula of a compound displays the elements that make up the compound in their simplest possible integer ratio.
Recommended Video for you:
How To Calculate Empirical Formula?
The empirical formula for any compound can be determined in a few easy steps. The procedure involves finding the amount of each element in the compound and converting that amount to moles, followed by individual mole ratio calculations. Once the individual mole ratios are calculated, the ratios can be converted to whole numbers (if they aren’t already) and the empirical formula of the compound can then be written. Nonetheless, let’s take a more detailed look at the procedure to calculate the empirical formula of a compound.
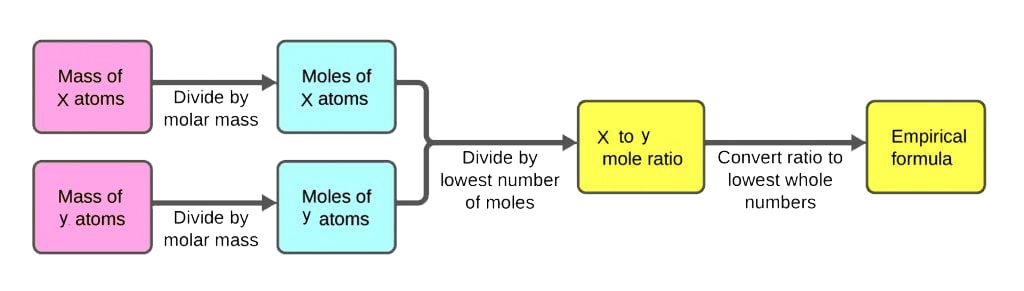
Step 1: Find The Mass (Amount) Of Each Element In The Compound
We start the procedure by finding the exact amount (in grams) of each element that makes up the compound being studied. Let’s assume that the compound whose empirical formula is to be found is XaYbZc. Chemical analysis of the compound XaYbZc yields information regarding the amount of each element (X, Y and Z) in percentage form. We convert these percentage values into simple numbers by assuming that we have 100g of the sample compound.
Let’s say that the results of a chemical analysis reveal that the compound XaYbZc has 40.0% of X, 6.7% of Y, and 53.3% of Z. If we have 100 grams of the sample compound, that would imply that X is 40 g, Y is 6.7 g and Z is 53.3 g.
We only need to perform this step when the composition of the compound is provided in percentage form. If the amount of each element is already available in grams, this step doesn’t need to be performed.
Step 2: Convert The Amount Of Each Element To Moles
In Step 2, we find the number of moles of each element using the mass values found in the previous step. In case you’ve been living under a rock, moles are a unit of measurement used to measure the quantities of small particles, such as atoms, molecules, electrons, protons, neutrons, etc. One mole of an element means that about 6.022×1023 atoms of the element are present. The formula to find the number of moles of an element from its amount is:
Number of moles = Amount of the element present (in grams) / Molar mass of the element
Coming back to our sample compound… the molar mass of X is 12.0107 g/mol, Y is 1.00784 g/mol and Z is 15.999 g/mol. (Note: One can find the molar mass of any element by performing a simple Google search.)
The number of moles are as follows: moles of X are 40/12.o1 = 3.33 mol; moles of Y are 6.7/1.00784 = 6.6 mol; and the moles of Z are 53.3/15.999 = 3.33 mol.
Step 3: Calculate Mole Ratios Of Each Element
Now, look for the element with the least number of moles in the compound. From the previous step, we see that element X and Z both have 3.33 moles in the compound, whereas Y has 6.66 moles.
We can find the mole ratios of each element by dividing the individual mole values by the smallest mole value found. Here, the smallest value is 3.33 moles. Thus, we find:
Number of moles of X/3.33 = 3.33/3.33 = 1
Number of moles of Y/3.33 = 6.66/3.33 = 2
Number of moles of Z/3.33 = 3.33/3.33 = 1
Step 4: Convert Mole Ratios Into Whole Numbers
The penultimate step in the procedure is converting the mole ratios to whole numbers, in case they aren’t already. The mole ratios we found in the previous step are 1, 2 and 1, all of which are whole numbers. Thus, we can skip this step and proceed to the final step.
However, if the mole ratios weren’t whole numbers, multiplying them by an integer would provide the desired values. Let’s say the mole ratios for another compound are 1.5 and 2. 1.5 isn’t a whole number and must be converted into one. Simply multiplying it by 2 would give us a whole number.
Note: Make sure you multiply all the mole ratios by the integer, and not just the non-whole number mole ratio.
1.5 x 2 = 3 and 2 x 2 = 4
Step 5: Write The Empirical Formula
Now that we have all the required data in our hands, it’s time to write the empirical formula for the compound XaYbZc. Write down the symbol of each element in the compound, followed by its mole ratio as a subscript.
For our sample compound, the empirical formula is X1Y2Z1 or simply XY2Z.
Also Read: Grams To Moles: How To Convert Grams To Moles?
Final Words
The sample compound XaYbZc that we’ve been using as an example was actually glucose. The molecular formula for glucose is C6H12O6, but as we just concluded in the final step of the procedure, its empirical formula is CH2O.
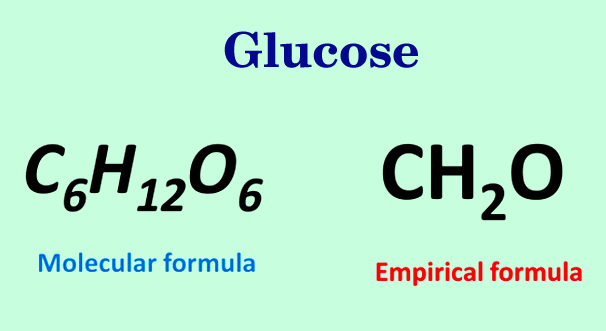
Thus, it can be seen that empirical formulas only convey information regarding the ratios in which the elements are present, not the actual number of atoms in the compound. Information regarding the actual number of atoms of an element present in a compound is provided by its molecular formula. To determine the molecular formula of a compound, multiply the subscripts of each element in the empirical formula by the ratio of molecular weight to empirical formula mass.
Also Read: How To Balance A Chemical Equation?