Table of Contents (click to expand)
‘Getting toasted’ means that a piece of bread is exposed to a considerable amount of concentrated heat inside the toaster. The resultant brown color is the consequence of the Maillard reaction that occurs when bread is radiated with heat.
Bread, to be sure, is loved by nearly everyone. There are many reasons for its popularity, but for me, it’s indispensable because you can make so many delicious dishes with bread as a key ingredient; its culinary applications are too numerous to list. However, let’s talk about one of the most popular options that bread offers to humanity – toast!
Why does it appear and taste the way it does?
Before you put a slice of bread in a toaster, it has its usual color, i.e., white or brown, but when it pops out of the toaster, it assumes a brownish hue. Not only that, it becomes much more delicious than its basic state. Why does that happen?
Recommended Video for you:
Why Does Bread Turn Hard When Toasted?
Bread attains a hardened texture after being put in a toaster, because, well… it ‘gets toasted’. In technical terms, ‘getting toasted’ means that it’s exposed to a considerable amount of concentrated heat inside the toaster. The resultant brown color is the consequence of the Maillard reaction (more details here) that occurs when bread is radiated with heat.

When you put bread in the toaster, the dry heat from the toaster eliminates every trace of moisture from within the slice. As a result, its elasticity disappears. Poof!
This simple physical phenomenon is why toasted bread is harder than regular, plain bread.
Also Read: Does Burnt Toast Have The Same Amount Of Calories As Regular Toast?
Why Does Bread’s Taste Change Upon Toasting?
Now you know why bread gets hard when toasted, but why does it change color and flavor?
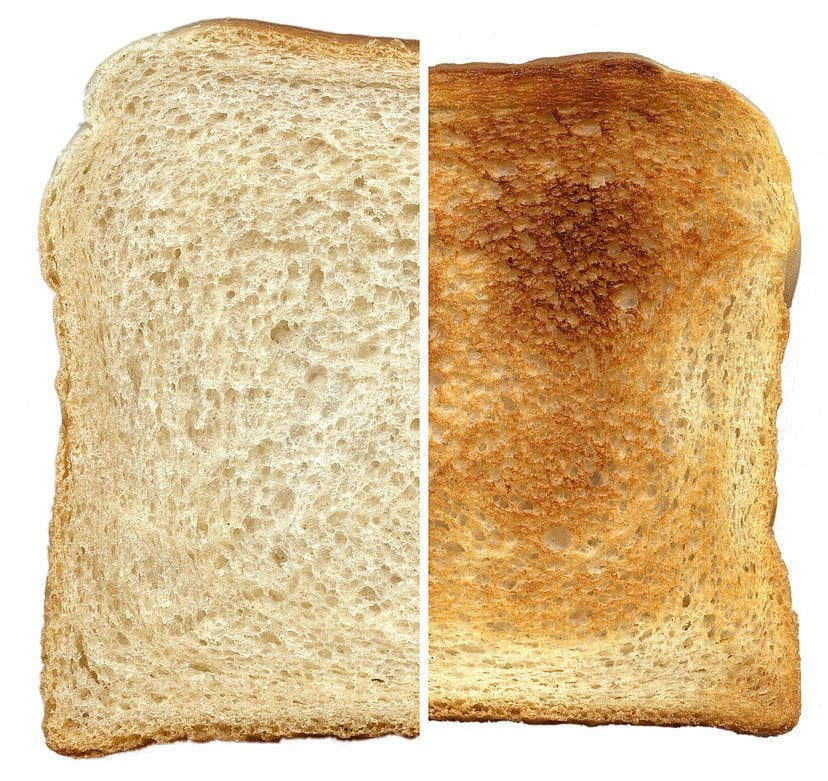
These changes are due to the Maillard reaction mentioned above. In basic terms, it’s a chemical reaction that consists of reducing sugars and amino acids that gives bread its characteristic flavor. You can find applications of this reaction in many day-to-day food items, including condensed milk, roasted coffee, black garlic, french fries and so on.
Bread contains proteins and carbohydrates. Due to the Maillard reaction, the outer layer of carbohydrates and amino acids combine, resulting in a caramelized brown color and that signature flavor of toasted bread. For all of this to happen, you need to heat the bread to temperatures in the range of 120 to 160 degrees Celsius (250-350 Fahrenheit), which is provided by the toaster. You insert a regular, relatively bland slice of bread into the toaster, and out comes a crispy, crunchy and tasty alternative!
Humans have known for centuries that foods take on new aromas and tastes when they turn brown, but it was only in the last century that a chemist Louis-Camille Maillard figured out why. He noted that under high heat, carbon atoms reduce sugars such as lactose, glucose and fructose, and then combine with nearby amino acids to form new compounds.
It’s interesting to note that while the Maillard reaction occurs fairly quickly when you cook, it can also occur over longer periods of time in foods stored at lower temperatures. For instance, the flavors that ripening cheese develops, are, in part, due to the Maillard reaction. Another example of the Maillard reaction taking a long time is Serrano ham—a raw meat that is dry-cured for a few months to bring out amazing flavors!
Dry Heat Is The Key!
For the Maillard reaction to occur properly, it’s important that the applied heat is ‘dry’ in nature. In other words, no water!
If you want to master the Maillard reaction and use it to your advantage when cooking, you need to create the right ‘environment’ around your food to let chemistry do its thing and bring out the best flavors. You see, the Maillard reaction moves fastest at high temperatures with little moisture, plus neutral to slightly alkaline conditions.
If you use excess water, it either slows or completely halts the Maillard reaction, which is the main reason why boiling doesn’t cause browning in foods, but grilling does.
Therefore, it’s important to get the right conditions in place for the Maillard reaction to work wonders on your recipes.
Next time you crunch into a delicious slice of toast, remember that such a pleasant taste is actually the result of chemical reactions occurring at a microscopic level within the individual particles of bread!
Also Read: Why Does Meat Taste Like Meat?












